Biotech Stock Roundup: Regulatory Updates From NVAX, BBIO Down on Drug Failure & More
Regulatory and other pipeline updates from NVAX and BBIO are among a few key highlights from the biotech sector during the past week.

This story originally appeared on Zacks
It was a low-key week for the biotech sector with a few regular pipeline and regulatory updates. Nonetheless, updates on COVID-19 vaccines and treatments continue to be in the spotlight amid the rapidly spreading Omicron variant.
Recap of the Week’s Most Important Stories:
Regulatory Update From Biogen:Biogen BIIB and partner Eisai announced that the FDA granted a Fast Track designation to lecanemab, an investigational anti-amyloid beta (Aβ) protofibril antibody, for the treatment of early Alzheimer’s disease (AD). The designation is designed to facilitate development and acceleration of the review of drugs to treat serious conditions and fill an unmet medical need or offer a potential advantage over the existing treatments. In September 2021, Eisai initiated a rolling submission of a biologics license application (BLA) for lecanemab to the FDA under the accelerated approval pathway.
Earlier, Biogen and Eisai had announced that the First Committee on New Drugs (NDC) of the Pharmaceutical Affairs and Food Sanitation Council, which advises the Ministry of Health, Labour and Welfare (MHLW) in Japan, decided to continue its deliberations on the application for manufacturing and securing a marketing approval of their AD drug Aduhlem (aducanumab).
Biogen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Novavax Vaccine Updates:Novavax, Inc. NVAX and its India based partner Serum Institute of India Pvt. Ltd. (SII) announced that the regulatory body in India granted an emergency use authorization (EUA) to the recombinant nanoparticle protein-based COVID-19 vaccine with MatrixM adjuvant of the former. The vaccine will be manufactured and marketed in India by SII under the brand name Covovax.The vaccine was granted an EUA in many other countries.
Novavax had previously announced initial data from the evaluation of its COVID-19 vaccine NVX-CoV2373 against the Omicron variant. Per the data, the vaccine’s two-dose regimen demonstrated cross-reactive immune responses against Omicron and other variants. These immune responses increased with the administration of a booster dose of NVX-CoV2373. Immune responses in adolescents were two- to four-fold higher than adults against a broad array of variants of interest and those of concern
Novavax also announced plans to begin clinical studies in the first quarter of 2022 on an Omicron-based COVID-19 vaccine.
BridgeBio Plunges Due to Heart Drug Failure: Shares of BridgeBio Pharma BBIO slumped significantly after the same announced top-line results from part A of ATTRibute-CM, an ongoing global phase II study investigating acoramidis for the treatment of symptomatic transthyretin (TTR) amyloid cardiomyopathy (ATTR-CM).
Data from the study demonstrated that after 12 months of treatment with acoramidis, the candidate failed to meet the study’s primary endpoint of improvement in the six-minute walk distance (6MWD) test relative to placebo.
However, the candidate improved quality of life, also improved NT-proBNP and stabilized serum transthyretin (TTR) levels that are biomarkers for an increased risk of heart failure. The independent data monitoring committee for the ATTRibute-CM study recommended BBIO to continue the study despite the disappointing performance of the six-minute walk test. BridgeBio and the committee believe that acoramidis holds potential to show improvement in all-cause mortality and cardiovascular hospitalizations after 30 months of treatment.
Gilead’s Data on Veklury:Gilead Sciences, Inc. GILD announced full results from a phase III investigational study evaluating the efficacy and safety of a three-day course of Veklury (remdesivir) for intravenous (IV) use. This is regarding the treatment of COVID-19 in non-hospitalized patients who are at a high risk of disease progression.
The participants who received Veklury treatment in the randomized, double-blind, placebo-controlled trial, experiences an 87% reduction in risk for the composite primary endpoint of COVID-related hospitalization or all-cause death by day 28. An 81% reduction in the risk for the composite secondary endpoint of COVID-related medical visits due to COVID-19 or all-cause death by day 28 compared to placebo was also observed from the study data.
The study includes new subgroup analyses, which showed consistent efficacy of Veklury for patients, irrespective of their key risk factors emanating from a severe condition of COVID-19. Per Gilead, data was submitted to the FDA for the potential use of Veklury in earlier stages of the infection, including prior to hospitalization.
Performance
The Nasdaq Biotechnology Index has lost 1.66% in the past four trading sessions. Among the biotech giants, Regeneron has gained 5.22% during the period. Over the past six months, shares of Regeneron have surged 17.65%. (See the last biotech stock roundup here: Biotech Stock Roundup: Biotech Stock Roundup: Regulatory Updates From BIIB, AMGN and NVAX & More)
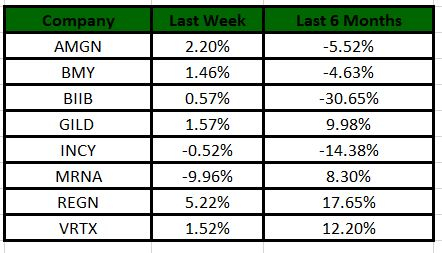 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
What’s Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don’t buy now, you may kick yourself in 2022.
Click here for the 4 trades >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
BridgeBio Pharma, Inc. (BBIO): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
It was a low-key week for the biotech sector with a few regular pipeline and regulatory updates. Nonetheless, updates on COVID-19 vaccines and treatments continue to be in the spotlight amid the rapidly spreading Omicron variant.
Recap of the Week’s Most Important Stories:
Regulatory Update From Biogen:Biogen BIIB and partner Eisai announced that the FDA granted a Fast Track designation to lecanemab, an investigational anti-amyloid beta (Aβ) protofibril antibody, for the treatment of early Alzheimer’s disease (AD). The designation is designed to facilitate development and acceleration of the review of drugs to treat serious conditions and fill an unmet medical need or offer a potential advantage over the existing treatments. In September 2021, Eisai initiated a rolling submission of a biologics license application (BLA) for lecanemab to the FDA under the accelerated approval pathway.
Earlier, Biogen and Eisai had announced that the First Committee on New Drugs (NDC) of the Pharmaceutical Affairs and Food Sanitation Council, which advises the Ministry of Health, Labour and Welfare (MHLW) in Japan, decided to continue its deliberations on the application for manufacturing and securing a marketing approval of their AD drug Aduhlem (aducanumab).






