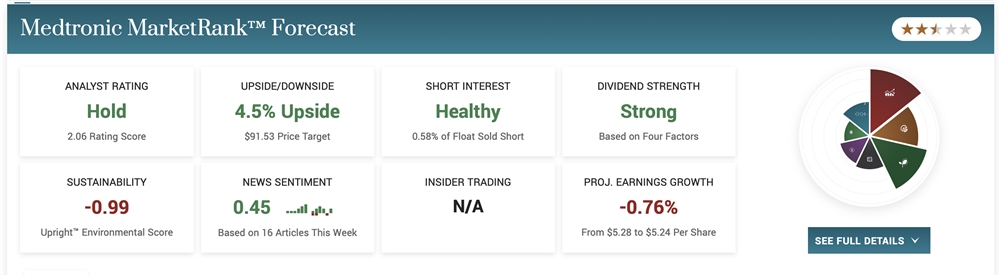
Medtronic: Reversal In-Play For This High-Yield Stock
Medtronic is getting upgraded after the FDA approved the MiniMed 780G insulin pump, a revolutionary device for diabetics worldwide.
This story originally appeared on MarketBeat

The med-tech companies have gotten a lot of attention lately, and Medtronic (NYSE: MDT) is included. The rebound in procedures and healthcare services following the pandemic drives a rebound in the industry amplified by innovation. In the case of Medtronic, it just received approval for an insulin pump that will alter the lives of its users. The pump is called the Minimed 780G and has been available in the EU since 2020.
The pump is the 1st of its kind to include testing at 5-minute intervals to more closely mimic natural body responses to changes in sugar levels. The meal-detection feature adjusts and corrects insulin levels as needed and can keep a diabetic’s sugar level in range for 75% of the time and keep below-range events to less than 2% of the time. Those stats are better at night when users sleep, and the device is approved for children as young as 7. Medtronic expects to begin shipping the new units this summer.
“A lot can happen to blood sugars in the span of an hour or even just a few minutes, so we’ve designed our system for real life – the algorithm adapts to the user and helps compensate for everyday challenges that are quite common around mealtimes,” EVP and President of Medtronic Diabetes Que Dallara said.
The Analysts Put The Bottom In Medtronic Price Action
The analysts’ sentiment was already firming in Medtronic, but the activity took on a new tone following the 780G approval. Marketbeat.com has picked up 3 new commentaries, including 2 price target increases and 2 upgrades from Equal Weight to Overweight. Those come from Barclays and Wells Fargo, which view the approval as a significant milestone.
Raymond James, which did not issue commentary specific to Medtronic, issued a downgrade for competitor Tandem, calling the news unfortunate and a potential headwind for sales and advancement. The consensus price target for Medtronic is still low, only about 3% above the current price action. Still, it has begun to trend higher, and several of the most recently issued are well into double-digit territory.
Medtronic reports at the end of May and is expected to issue solid results. The 780G won’t impact this quarter’s results, but it may begin to show up in the guidance, and it will certainly show in the analysts’ consensus targets for Q1 and Q2. The US insulin pump market is worth upwards of $5 billion this year and is expected to grow at a high-single-digit CAGR for the next few years at least. Medtronic is positioned to grow along with the market and take market share, which is the real win.
Institutions Are Buying Medtronic’s 3% Yield
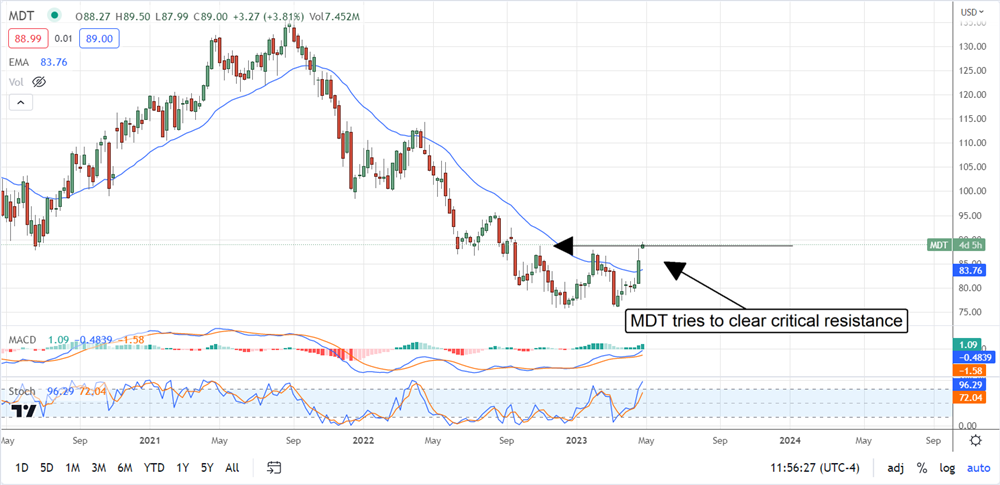
The institutions own about 80% of the stock and have bought it over the past 2 years. The quarter-to-quarter action is mixed, but they have been buyers on balance for most quarters and picked up the pace of buying in Q1. This has the stock bottoming and is now on the cusp of reversal. The post-release action has the market up more than 3.5% and at the highest level in over 9 months. The next hurdle is at the current level, and if it can be overcome, a sustained rally may form. If not, this stock will remain range bound at current levels.

The med-tech companies have gotten a lot of attention lately, and Medtronic (NYSE: MDT) is included. The rebound in procedures and healthcare services following the pandemic drives a rebound in the industry amplified by innovation. In the case of Medtronic, it just received approval for an insulin pump that will alter the lives of its users. The pump is called the Minimed 780G and has been available in the EU since 2020.
The pump is the 1st of its kind to include testing at 5-minute intervals to more closely mimic natural body responses to changes in sugar levels. The meal-detection feature adjusts and corrects insulin levels as needed and can keep a diabetic’s sugar level in range for 75% of the time and keep below-range events to less than 2% of the time. Those stats are better at night when users sleep, and the device is approved for children as young as 7. Medtronic expects to begin shipping the new units this summer.